Definition of Carbohydrates
- Carbohydrates are carbon, hydrogen, as well as oxygen-containing compounds.
- Hydrogen atoms have twice the number of carbon or oxygen atoms.
- Cx(H2O)y is a carbohydrate’s general formula.
- They are energy sources (e.g. glucose), energy stores (e.g. starch as well as glycogen), as well as as structural elements (e.g. cellulose inside plants as well as chitins inside insects).
- Polymers are the most common type of carbohydrate.
- Polymers are lengthy chains of monomers that make up huge, complex compounds.
- Monomers are molecular units that are tiny as well as simple.
- Monosaccharides, disaccharides, as well as polysaccharides are the three types of carbohydrates available.
Monosaccharides – Structure, Properties and Examples
- Monosaccharides are simple sugars that include one atom of oxygen plus two atoms of hydrogen for each atom of carbon.
- Their general formula is (CH2O)n.
- Monosaccharides help to lower blood sugar levels.
- Benedict’s test is a procedure for lowering blood sugar levels.
- Sweet-flavored sugars that are soluble in water but not soluble in non-polar solvents.
- These could be seen in straight, ring, or cyclic chains.
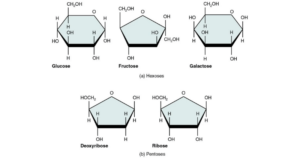
- Based on the number of carbon atoms within every molecule, these are classified as trioses (3C), tetroses (4C), pentoses (5C), hexoses (6C), heptoses (7), and so on.
- The names of all sugars end in –ose.
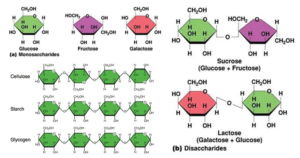
- Examples include Glyceraldehyde (triose), Erythrose (tetrose), Ribose (pentose), Glucose (hexose), Fructose (hexose), Galactose (hexose), Sedoheptulose (heptose), and others.
- They provide energy to the body during respiration.
- They are necessary for the formation of large molecules.
Disaccharides – Structure, Properties, and Examples
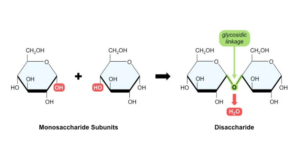
Disaccharides are formed by the condensation process of two monosaccharides.
- The condensation reaction includes the production of a new chemical bond among two molecules, that results in the discharge of a water molecule.
- A glycosidic bond is formed among two monosaccharides. Once carbon 1 on one monosaccharide connects carbon 4 on another monosaccharide, a 1,4-glycosidic bond is formed.
Examples of Disaccharides
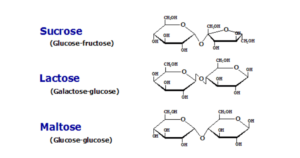
- Maltose is composed of two -glucose molecules linked together by a glycosidic bond.
- Sucrose is made whenever a glucose molecule as well as a fructose molecule combine in a condensation event.
- Lactose is composed of a glucose molecule as well as a galactose molecule.
- Sucrose is a non-reducing sugar.
- The hydrolysis reaction breaks disaccharides into two monosaccharides by dissolving the glycosidic bond with the addition of water molecules. Water’s hydroxyl group (-OH) and hydrogen (-H) contribute in the breakdown of the glycosidic bond.
- Sucrose is a transport sugar, whereas Lactose is a sugar present in milk, that is an essential nutrient of young mammals.
Polysaccharides- Structure, Properties, and Examples
- Polysaccharides are polymers generated by condensation processes which connect several monosaccharide molecules (more than two).
- Oligosaccharides are molecules which contain 3-10 sugar units, while true polysaccharides contain 11 or more monosaccharides.
- Polysaccharides do not have a sweet flavour.
- The bulk of polysaccharides don’t dissolve in water because of their massive molecules.
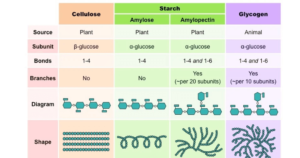
- Homopolysaccharides (Starch) are polysaccharides created entirely of one kind of monomer, whereas heteropolysaccharides are polysaccharides composed of than one monomer (Hyaluronic acid).
- Long chains of -glucose make up starch (Amylose as well as Amylopectin). Glycogen is composed of α-glucose molecules connected by glycosidic linkages. Many β-glucose molecules are connected by glycosidic linkages between carbon 1 as well as carbon 4 in cellulose.
- Starch is the main energy storage material in plants. Glycogen is the most common form of energy storage in mammals. Plant cell walls are primarily made up of cellulose.
- An Iodine test is used to detect starch.
Click Here for Complete Biology Notes
Carbohydrates Citations
- 2015. A-Level Biology Exam Board: AQA. Complete Revision and Practice. Original material by Richard Parsons.
- Glenn Toole and Susan Toole. 2015. AQA Biology for A-Level. 2nd Edition. Oxford University Press.
- Mary Jones, Richard Fosbery, Jennifer Gregory and Dennis Taylor. 2014. Cambridge International AS and A Level Biology Coursebook. 4th Edition. Cambridge University Press.
- Mary Jones. 2010. Cambridge International A/AS-Level Biology Revision Guide. Hodder Education.
- Sue Hocking, Frank Sochacki and Mark Winterbottom. 2015. OCR AS/A level Biology A. 2nd Edition. Pearson Education Limited
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results