Definition of Solute
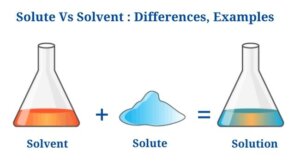
A solute is a material that can be mixed with a solvent to make a solution.
- The solute can exist in any of the three states of matter as a solid, liquid, or gas.
- In a homogeneous mixture, the solute completely dissolves in another substance, and the solute is evenly dispersed all through the solution.
- In a heterogeneous combination, the solute is not distributed equally all through the solution, and its concentration differs.
- The quantity of a solute in a solution is determined by its concentration in the solution. The concentration of solute in a solution is determined by the ratio of the amount of solute to the total volume of the solution.
- Solubility refers to the ability of solute particles to dissolve in a solvent. A solute’s solubility is determined by a variety of factors.
- In solids and gases, temperature has a direct impact on the solute’s solubility. Pressure, on the other hand, has no effect on the solubility of gases.
- Furthermore, solid particles’ ability to dissolve in a solvent is determined by their chemical structure. In a polar solvent, a polar solute dissolves, and vice versa.
- Solute particle size is critical in a solution because the solvent breaks them down and disperses them throughout the solution.
- The amount or volume of solute in practically all forms of solutions is less than that of the solvent.
- The boiling point of solute particles is higher than that of solvents.
- Salt in saltwater, protons in the cytosol, sugar in tea, and other solutes are examples.
Definition of Solvent
Throughout the making of a solution, a solvent is a material which dissolves the solute particles.
- The majority of solvents are in a liquid condition, while some are in a gas or solid state.
- The solvent disperses the bigger solute particles throughout the solution by breaking them down into smaller particles.
- The solvent is the solution’s medium, accounting for the majority of its volume.
- The quantity of solute which can be disseminated in the solvent is determined by the medium’s temperature.
- A solution is a homogeneous mixture with uniformly dispersed solute particles throughout the solvent. As a consequence, the concentration of solute in every volume of solvent in the solution is the same.
- Solute-solvent complexes, also known as solvates, arise when the solvent and solute in a solution reside in a single phase.
- When a solution is formed, many solvent particles surround the solute particle, transferring heat energy from the solvent to the solute and resulting in a more thermodynamically stable condition.
- The solubility of any solute in the solvent is determined by the polarity of the solvent particle.
- Water is a polar molecule which can dissolve a high number of solute particles and is also known as a universal solvent.
- The majority of solvents are divided into two types: polar and non-polar solvents. Amalgams are a special kind of mercury-based solvent.
- The solvent’s boiling point is lower than that of the solute.
- Water, hydrocarbons, alcohols, esters, and other solvents are examples.
Difference between Solute and Solvent
(Solute vs Solvent)
[ninja_tables id=”5428″]
Solute Examples
Salt in seawater is an example of a solute.
- The salt, NaCl, is an ionic molecule in that the negatively charged chloride ion is attracted by the slightly positively charged hydrogen atom of water.
- The sodium as well as oxygen atoms have a comparable affinity, which allows NaCl to break down into smaller particles, which are subsequently spread throughout the water.
- The surface area of the solute particle determines the range of solubility as well as time period.
- As a result, coarse salts dissolve less than finer salts with a larger surface area.
- There will be no salt crystals seen in the fluid once all of the salt has been dissolved.
- Also check out Lewis Acids as well as Bases.
In cytosol, there are protons
- A cell’s cytoplasm contains protons, or H+, which help to keep the pH of the solution stable.
- Such protons are drawn to the oxygen atom in water molecules as well as so serve an important role in molecular transmembrane transit.
- Water passes through the membranes, but protons do not. Water molecules can freely pass through the membrane as a result.
- A proton motive force is formed by the attraction between water molecules as well as protons.
- After which, the proton motive force can be employed to move a number of chemicals through the membrane.
Solvent Examples
Water is an example of a solvent.
- Because it dissolves a wide range of solute particles, water is regarded as a universal solvent.
- Water is the foundation of numerous biological solutions that transport and transport essential particles all over the body.
- Water is a polar solvent with a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atom.
- Water molecules’ polarity makes them particularly compatible with a variety of solute molecules.
- Seawater is very crucial instance of water as a solvent. Large amounts of salt dissolved in water are carried by seawater.
oil
- Oil also works as a solvent in cooking, preventing polar as well as non-polar solutes from adhering to the pan.
- Hot oil forms a solution which can be used to cook other dishes.
- Oil contains a solute that can be added to the food being prepared.
- Oil is an organic chemical which acts as a non-polar solvent, allowing non-polar solute molecules to disperse all over the solution.
- Vegetable oil is a non-volatile organic compound (VOC) with a high dissolving power as well as flash point, as well as low toxicity as well as minimal environmental impact when compared to conventional petroleum solvents.
Solute Vs Solvent Citations
- https://brainly.com/question/7437740
- https://biologydictionary.net/solute/
- http://biologyaspoetry.com/terms/proton_motive_force.html
- https://www.youtube.com/watch?v=E-M8JcYr2d4
- https://www.wikihow.com/Calculate-the-Concentration-of-a-Solution
- https://www.quora.com/What-is-the-difference-between-a-solute-solvent-and-solution
- https://www.khanacademy.org/science/biology/water-acids-and-bases/hydrogen-bonding-in-water/a/water-as-a-solvent
- https://web.viu.ca/krogh/chem331/SOLUBILITY%202010.pdf
- https://quizlet.com/11495145/chemistry-solutions-flash-cards/
- https://physics.stackexchange.com/questions/198213/how-does-the-dissolution-of-salt-affect-the-solution-density
- https://pharmlabs.unc.edu/labs/solubility/structure.htm
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example