What is Acetamide Utilization Test ?
Acetamide Utilization Test: One of several biochemical assays used to identify aerobic organisms is the acetamide consumption test. The acetamide test is used to assess an organism’s ability to deamidate Acetamide. Based on its usage, Acetamide is used in qualitative processes to differentiate non-fermentative, gram-negative bacteria, primarily Pseudomonas aeruginosa.
- This test is based on the organism’s use of Acetamide through the deamidation process.
- The acylamidase enzyme is present during the deamidation process.
- The difference between the fermentative and oxidative groups of organisms is determined using an acetamide consumption test. On the basis of their ability to utilize Acetamide, this has been widely used to differentiate or identify Gram-negative, non-fermentative bacteria such as Pseudomonas aeruginosa.
- The use of Acetamide by the organism cultured on the medium is studied using a medium with Acetamide as the only source of carbon. The result of the test is determined by the change in color of the media after growth.
- The citrate utilization test is similar to the acetamide utilization test in that it identifies the organism based on its capacity to use citrate as a sole source of carbon. This is used to identify organisms that are members of the Enterobacteriaceae family.
Objectives of Acetamide Utilization Test
To distinguish bacteria based on their capacity to use Acetamide as their only carbon source. To distinguish P. aeruginosa from other Gram-negative rods that do not ferment glucose.
Principle of Acetamide Utilization Test
- Acetamide agar is used to test an organism’s capacity to use Acetamide as a sole source of carbon after deamidation.
- The medium is made up of Acetamide, which is the only carbon source, and inorganic ammonium salts, which are the only nitrogen source.
- The organism’s growth on the acetamide agar indicates a positive acetamide utilisation test.
- The enzymatic action of acylamidase results in the degradation of ammonium salts into ammonia during a bacterium’s metabolism of Acetamide.
- The ammonia released thereby raises the medium’s alkalinity. The bromthymol blue indicator in the medium turns from green to blue as the pH of the medium changes, indicating a positive test.
- The colour of the medium remains the same in the absence of ammonia emission, suggesting a negative result.
- Assimilation of Acetamide can sometimes result in a yellow tint, which can be misinterpreted as a favorable result.
However, only a few species are capable of deamination digesting of Acetamide. As a result, this test is frequently used to distinguish Pseudomonas aeruginosa from other Gram-negative rods that do not ferment glucose.
Media used in Acetamide Utilization Test
The following is a list of the ingredients in Acetamide Agar:
|
S.N |
Ingredients |
Gram/liter |
|
1. |
Bromothymol blue |
0.08 |
|
2. |
Magnesium sulfate |
0.2 |
|
3. |
Potassium phosphate, dibasic |
1.0 |
|
4. |
Ammonium phosphate, monobasic |
1.0 |
|
5. |
Sodium chloride |
5.0 |
|
6. |
Acetamide |
10.0 |
|
7. |
Agar |
15.0 |
|
Final pH at 25°C: 6.8 ±0.2 |
||
|
Store at 2°C to 8°C. |
||
Acetamide agar is obtainable commercially also and might have a dissimilar composition
|
S.N |
Ingredients |
Gram/liter |
|
1. |
Phenol red |
0.03 |
|
2. |
Dextrose |
0.2 |
|
3. |
Yeast extract |
0.5 |
|
4. |
Potassium dihydrogen phosphate |
1.0 |
|
5. |
Acetamide |
3.0 |
|
6. |
Sodium chloride |
5.0 |
|
7. |
Bacteriological Agar |
15.0 |
|
Final pH at 25°C: 6.8 ±0.2 |
||
Used supplies or equipment
- Inoculating loops or sticks that are sterile.
- Preheat the oven to 350 degrees Fahrenheit.
Acetamide Utilization Test Procedure
Preparation of the media
- 24.7 grams of dehydrated powder or lab-prepared media are mixed with 1000 milliliters of distilled or deionized water in a beaker.
- The suspension is then brought to a boil to dissolve the medium completely.
- After that, the dissolved medium is poured into tubes and sterilized in an autoclave for 15 minutes at 15 lbs pressure (121°C).
- After the autoclaving process is completed, the tubes are removed and cooled to a temperature of around 40-45°C in a tilted posture. To obtain butts of 1.5–2.0 cm depth, the posture should be maintained.
Utilization test
- A sterile inoculating needle is used to extract a well-isolated colony from an 18-24 hour culture.
- By streaking the surface of the slant, the acetamide agar tubes are infected. With the loop or the inoculating stick, streak the slant back and forth.
- To maintain appropriate aeration, the test tube caps should be left unfastened.
- The tubes are then incubated aerobically for up to 7 days at 35-37°C.
- Before disregarding the result as a negative, the test tubes should be inspected everyday for four days and then seven days.
Result of Acetamide Utilization Test
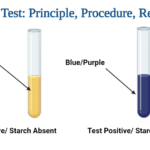
Growth and a change in hue from green to bright blue along the slant indicate a favorable test. There is no growth, no color change, and the slant remains green in a negative test.
Bacterial control
Two distinct species can be used as a positive and negative control for the acetamide utilization test as a type of quality control.
|
Control |
Incubation |
Results |
|
P. aeruginosa |
Aerobic incubation for 24-48 hours at 33-37°C. |
Acetamide positive (growth; blue color)
|
|
Escherichia coli |
Aerobic incubation for 24-48 hours at 33-37°C. |
Acetamide negative (no growth; green color) |
Uses of Acetamide Utilization Test
- The Acetamide Utilization Test evaluates an organism’s capacity to use acetamide as its only supply of carbon.
- It is also employed as a qualitative test to distinguish between the fermentative and oxidative groups of Gram-negative bacteria.
- Acetamide agar is also used to isolate P. aeruginosa as a selective medium.
- The ability of acetamide to operate as a carbon source in the absence of peptone or other protein sources was tested using a modified Simmon Citrate agar medium.
Limitations of Acetamide Utilization Test
- A positive test could be indicated by growth on the slant without an accompanying colour change. The test should be repeated with less inoculum if the agar does not turn blue after more incubation.
- A negative test does not rule out the possibility of P. aeruginosa being identified. Other tests should be run to confirm the presence of P. aeruginosa.
- Only around 38% of non-pyocyanin-producing P. aeruginosa strains generate a positive result in this test; hence, additional biochemical tests for conclusive identification should be undertaken.
- Since the test demands an aerobic atmosphere, the slant should not be stabbed.
- As there is a risk of media carryover with broth cultures, inoculums should not be taken from them.
- To prevent the transfer of chemicals from prior media, a mild inoculum should be used.
Acetamide Utilization Test Citations
- Oberhofer, T. R., & Rowen, J. W. (1974). Acetamide agar for differentiation of non-fermentative bacteria. Applied Microbiology, 28(4), 720–721.
- Aetamide Agar. Thermo Fisher Scientific Inc.
- Bailey and Scott’s Diagnostic Microbiology. Elsevier.
Related Posts
- Anisocytosis: Definition, Types, Causes, Symptoms, Treatment
- Endospore Staining: Principle, Procedure, Reagents, Results
- Flow Cytometry: Overview, Principle, Steps, Types, Uses
- Northern Blot: Overview, Principle, Procedure and Results
- MPV Blood Test: Calculation, High and Low MPV Value, Results
- Latex Agglutination Test: Objectives, Principle, Procedure, Results
- Iodine Test: Definition, Objective, Principle, Procedure, Results
- Eosin Methylene Blue (EMB) Agar
- Biuret Test for Protein: Purpose, Objectives, Principle, Procedure, Reagents
- Streak Plate Method: Meaning, Principle, Methods, Importance, Limitations
- Bile Solubility Test: Objective, Principle, Procedure, Results, Uses
- Butyrate Disk Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Beta Lactamase Test: Objective, Principle, Procedure, Results, Limitations
- Bacitracin Susceptibility Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Bile Esculin Test: Objective, Principle, Procedure, Result, Uses, Limitations