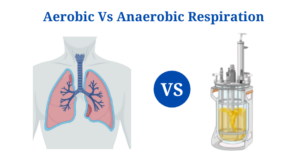
Definition of Aerobic Respiration
Aerobic respiration is a series of metabolic events that occur in the presence of oxygen in order to transform chemical energy into ATPs in a cell.
- Except for certain early prokaryotes, all plants, animals, birds, and humans use aerobic respiration.
- In aerobic respiration, oxygen serves as an electron acceptor, allowing ATPs to be produced more efficiently and quickly.
- The energy of the double bond in oxygen is higher than that of other bonds, allowing for the production of more ATPs.
- After glycolysis, it is the preferred method of pyruvate breakdown, in which the pyruvate enters the mitochondria and is totally oxidised during the Kreb’s cycle.
- The oxidation of carbohydrates is accomplished through aerobic respiration, but products from fats and proteins are also utilised as reactants.
- Aerobic respiration produces carbon dioxide gas and water, as well as the energy required to add a third phosphate group to ADP and generate ATP.
- Other energy-dense molecules, such as NADH and FADH2, are transformed to ATP by an electron transport chain that includes oxygen and protons.
- The majority of ATPs are produced during aerobic respiration during oxidative phosphorylation, which uses the energy of an oxygen molecule to pump protons out of the membrane.
- Aerobic respiration causes complete carbohydrate oxidation, which occurs in the mitochondria of eukaryotic cells since the enzymes required for the process are present there.
Definition of Anaerobic Respiration
Anaerobic respiration is a type of cellular respiration in which neither oxygen nor pyruvate derivatives serve as a high-energy electron acceptor.
- The electron acceptor in anaerobic respiration can be sulphate ion (SO4–), nitrate ion (NO3–), or a variety of other compounds.
- Methanogens are archaea that consume carbon dioxide as an electron acceptor and produce methane as a by-product.
- Another type of purple sulphur bacterium uses sulphate as an electron acceptor, resulting in the production of hydrogen sulphide as a by-product.
- Because these organisms live in low-oxygen conditions, anaerobic pathways are used to break down chemical fuels.
- Anaerobic respiration is similar to aerobic respiration in that the molecules join the electron transport chain, where the electrons are passed to the ultimate electron acceptor.
- Anaerobic respiration’s final electron acceptors have a lower reduction potential than oxygen molecules, resulting in less energy production.
- Anaerobic respiration, on the other hand, is necessary for carbon, nitrogen, and sulphur biogeochemical cycles.
- In anaerobic respiration, the nitrate that works as an electron acceptor creates nitrogen gas as a by-product, and this is the only way for fixed nitrogen to reach the environment.
- Fermentation is an anaerobic respiration pathway in which glycolysis is the only energy extraction pathway and the pyruvate is not further oxidised by the citric acid cycle.
- During fermentation, the energy-rich molecule NADH is also not used.
- Anaerobic respiration occurs in a variety of settings, including freshwater, soil, and deep-sea surfaces. Because oxygen cannot readily diffuse through their surface, certain bacteria in oxygenated settings use anaerobic respiration.
- The cytoplasm of the prokaryotic cell is where anaerobic respiration and fermentation take place.
- During acute contraction and relaxation, anaerobic respiration and fermentation activities occur in muscle cells.
- Only two ATPs are gained per glucose molecule during fermentation.
Difference Between Aerobic And Anaerobic Respiration
(Aerobic Respiration vs Anaerobic Respiration)
| Basis for comparison | Aerobic respiration | Anaerobic Respiration |
| Definition | Aerobic respiration is a series of metabolic events that occur in the presence of oxygen in order to transform chemical energy into ATPs in a cell. |
Anaerobic respiration is a type of cellular respiration in which neither oxygen nor pyruvate derivatives serve as a high-energy electron acceptor. |
| Overall equation |
C6H12O6 + 6O2 + 6CO2 + 6H2O + energy is the overall equation for aerobic respiration. |
C6H12O6 C2H5OH + CO2 + energy is the general equation for anaerobic respiration. |
| Presence of Oxygen |
In the presence of oxygen, aerobic respiration occurs. |
Anaerobic respiration occurs when there is a lack of oxygen in the surroundings. |
| Exchange of gases |
During aerobic respiration, oxygen is received and carbon dioxide is exhaled, resulting in a gas exchange. |
Anaerobic respiration does not include the exchange of gases. Sulfur and nitrogen gases, for example, are released by some organisms. |
| Location | After glycolysis, aerobic respiration occurs in the mitochondria of eukaryotes and the cytoplasm of prokaryotes. |
Anaerobic respiration takes place only in a cell’s cytoplasm. |
| End products |
Carbon dioxide, water, and energy are the end products of aerobic respiration. |
Acids, alcohols, gases, and energy are the end products of anaerobic respiration. |
| Energy produced | During aerobic respiration, a total of 38 ATPs are created, with some of them being lost in the process. |
During anaerobic respiration, only two ATPs are generated. |
| Reactants |
Carbohydrates and oxygen are required for aerobic respiration to occur. |
Along with the carbohydrates, other electron acceptors such as sulphur and nitrogen are necessary. |
| Oxidation |
During aerobic respiration, carbohydrates are completely oxidised. |
During anaerobic respiration, carbohydrates are incompletely oxidised. |
| Nature of the process | In comparison to anaerobic respiration, aerobic respiration lasts longer. |
Anaerobic respiration lasts for a shorter period of time than aerobic respiration. |
| Occurs in |
Most higher creatures, such as plants and animals, use aerobic respiration. |
In primordial prokaryotes, anaerobic respiration occurs. In humans, anaerobic respiration occurs in muscle cells during severe motions. |
Click Here for Complete Biology Notes
Aerobic Respiration Examples
Respiration in humans
- The process of cellular respiration in humans is aerobic respiration, in which the organism obtains its energy from the complete oxidation of glucose.
- It starts in the cell’s cytoplasm, and the products are subsequently transported to the mitochondria, where they undergo additional reactions.
- The lungs take oxygen, which is then stored in red blood cells. The oxygen is subsequently delivered to the cells that need it.
- The glucose is then oxidised, generating carbon dioxide gas in the process.
- The key metabolic processes for the oxidation of carbohydrates to liberate energy are included in cellular respiration in humans.
Anaerobic Respiration Examples
(a) In muscles, lactic acid is produced.
- The muscles in our bodies cannot obtain enough oxygen during hard exercise, therefore they execute more glycolysis than the body can transfer oxygen to the electron transport chain.
- Due to a lack of oxygen in our muscles, anaerobic respiration occurs.
- As a result, anaerobic respiration takes place instead of aerobic respiration, resulting in the creation of lactic acid.
- Lactic acid fermentation is a type of anaerobic respiration that yields just two ATPs per glucose molecule.
- Lactic acid fermentation can be written as follows:
C6H12O6 → C3H6O3 + energy
- Lactic acid fermentation in muscles causes lactic acid to build up in the tissues, resulting in aching muscles.
- Anaerobic respiration produces less energy per glucose molecule than aerobic respiration, resulting in fatigue and shortness of breath.
How to increase Brain Power – Secrets of Brain Unlocked
(b) Alcoholic fermentation by yeasts
- Fermentation is a type of anaerobic respiration that involves the consumption of anaerobic organisms such as yeasts.
- Yeasts conduct anaerobic respiration when carbohydrate-rich items are bottled with them, ensuring that the oxygen content in the bottle is kept to a minimum.
- As a result, the yeast transforms carbohydrates into ethyl alcohol, resulting in fermentation.
- However, the yeasts are toxic to the alcohol created in the bottle, which is why they begin to die as the alcohol concentration rises.
- Only approximately 30% of alcohol can be produced with yeasts, with larger concentrations achieved through the distillation process.
- Fermentation, like lactic acid fermentation, produces only two ATPs as energy.
- Fermentation’s total reaction can be written as:
C6H12O6 → C2H5OH + CO2 + energy
(c) Methanogen fermentation
- Methanogens are archaea-related prokaryotes.
- Because they make methane as a by-product of oxidising carbohydrates in the absence of oxygen, these organisms are called methanogens. Methanogenesis is the name for this process.
- This is another type of fermentation that produces methanol, a distinct sort of alcohol. Methanol poisoning is another name for this phenomenon.
- Methanogens (such as Methanosarcina barkeri) oxidise plant cellulose to make methanol rather than ethyl alcohol, as yeasts do.
- Some persons may have nerve damage or even death as a result of methanol intoxication.
- The following is the overall response of methanol production:
C6H12O6 → CH3OH + CO2 + energy
(d) Fermentation of propionic acid in cheese
- Propionic acid fermentation occurs when bacteria (such as Propionibacterium shermanii) use carbohydrates such as lactose and glucose to create propionic acid and CO2.
- Swiss cheese is the most common example of this process in action.
- Because of the carbon dioxide gas created during this process, bubbles form in the cheese, as well as a particular flavour due to the carboxylic acid.
- This, like all other anaerobic respiration processes, occurs when there is no or little oxygen available.
- This process’s overall reaction is:
C12H22O11 → C3H6O2 + CO2 + energy
Citations
- https://quizlet.com/232774637/chapter-9-old-flash-cards/
- https://chem.libretexts.org/Bookshelves/Ancillary_Materials/lhrli@ucdavis.edu/Book%3A_Biology_for_Majors_I_(Lumen)/09%3A_Module_6%3A_Metabolic_Pathways/09.15%3A_Electron_Transport_Chain
- https://brainly.com/question/1620230
- https://brainly.com/question/1599790
- https://biologywise.com/function-of-nadh-fadh2
- https://biologywise.com/aerobic-anaerobic-respiration
- https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Boundless)/5%3A_Microbial_Metabolism/5.09%3A_Anaerobic_Respiration/5.9A%3A_Electron_Donors_and_Acceptors_in_Anaerobic_Respiration
- https://antranik.org/cell-respiration-part-1-anaerobic-respiration-glycolysis-and-fermentation/
- https://www.sciencedirect.com/topics/earth-and-planetary-sciences/methanogenesis
- https://www.sciencedirect.com/science/article/pii/0043135469900025
- https://www.scienceabc.com/pure-sciences/different-steps-cellular-respiration-definition-biology.html
- https://www.med-health.net/Aerobic-Respiration-Equation.html
- https://www.britannica.com/science/carbonic-acid
- https://www.bbc.co.uk/bitesize/topics/zvrrd2p/articles/ztdmrwx
- https://www.bbc.co.uk/bitesize/guides/z32797h/revision/3
- https://www.answers.com/Q/How_does_respiration_take_place_in_animals
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results