Definition of Aldehyde
An aldehyde is a type of organic chemical that has a functional group with the formula –CHO, with the carbonyl group double-bonded to oxygen.
- Aldehydes are created when a hydrogen atom is removed from an alcohol molecule.
- Chemical formula for aldehyde is R-CHO, where R is an alkyl group as well as the carbon atom is double-bonded to oxygen as well as single-bonded to hydrogen. Saturated, unsaturated, alicyclic, aromatic, or heterocyclic alkyl groups are all possible.
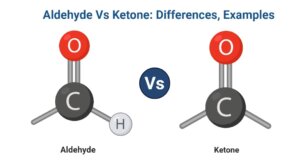
- The –CHO group, commonly known as the aldehyde group, is found near the carbon chain’s terminal.
- Aldehydes are reactive chemicals which can either be reduced to alcohols or oxidised to carboxylic acids.
- The majority of aldehydes have a pleasant odour as well as are produced from alcohols through dehydrogenation.
- The aldehydes are given the suffix –al in accordance with the IUPAC method, as well as there is no need for numbering since the aldehyde group is always at the end of the chain.
- Since of the presence of a hydrogen bond, the aldehyde group in aldehyde is polar, that affects the physical properties of the chemical.
- In analytical chemistry, there are a variety of tests as well as reactions that may be used to differentiate aldehyde from other organic molecules.
- Such tests are frequently predicated on the identification of the aldehyde group as part of chemical identification assays.
- In a chemical laboratory, tests such as Fehling’s as well as Tollen’s are used to distinguish between aldehydes as well as ketones.
- Aldehydes are organic chemistry building blocks that can be used to make a variety of different chemicals.
- Aldehydes are important in industry since they’re employed as solvents, fragrances, as well as flavouring agents. Some even find their way into colours, plastics, as well as pharmaceuticals.
Definition of ketone
The existence of a carbonyl group (CO) as a functional group distinguishes ketone from other chemical molecules.
- A carbon atom is double-bonded to oxygen in the carbonyl group, while the rest of the two bonds are to other carbon atoms in alkyl groups.
- Depending on the molecule, the alkyl group linked to the carbonyl group can be aliphatic, aromatic, or alicyclic.
- Ketone’s carbonyl group joins two alkyl groups as well as does not appear at the end of the chain.
- Ketones are further divided into groups based on their substituents as well as substituent equivalency.
- The nature of the alkyl groups linked to the carbonyl group determines whether ketones are symmetrical or asymmetrical. Diketones are carbonyl compounds that have two carbon atoms.
- Ketones are formed by reducing secondary alcohols as well as can then be oxidised to make carboxylic acids.
- Ketones are named by altering the suffix –ane to –anone, according to IUPAC naming guidelines. The location of the carbonyl group in the molecule must be indicated.
- In analytical chemistry, there are a variety of tests as well as reactions that may be used to differentiate ketone from other organic molecules.
- As part of the identification tests of various compounds, these assays identify the carbonyl group existing in the substance.
- Since ketones as well as aldehydes are structurally similar, analytical procedures are used to distinguish the two.
- In the chemical laboratory, tests such as that Fehling’s as well as Tollen’s are used to distinguish between aldehydes as well as ketones.
- Ketone molecules are essential since they are found in many sugars as well as other chemicals that are used as steroid hormones in medicine.
- The majority of ketones are found in nature, as well as only a small percentage of ketones can be produced in factories.
- Ketones, on the other hand, can be employed as intermediates in the construction of complex organic molecules.
Key Differences between Aldehyde and Ketone
(Aldehyde Vs Ketone)
[ninja_tables id=”5475″]
Aldehyde Examples
Formaldehyde
- The simplest aldehyde molecule is formaldehyde, that has the aldehyde group linked to hydrogen.
- Formaldehyde is made by oxidising methanol as well as is utilised in labs as an antiseptic, disinfectant, as well as general-purpose chemical reagent.
- It’s a colourless hazardous gas which can cause unconsciousness as well as other problems if inhaled over lengthy periods of time.
- Since the chemical rapidly polymerizes into paraformaldehyde, it is commonly maintained as formalin in aqueous form.
- Formaldehyde is a key chemical compound which serves as a precursor to a variety of organic molecules.
- Formaldehyde can be found in the natural world in a variety of places, including the upper atmosphere, living creatures, as well as space.
- Formaldehyde is used to make urea resin, polymethylene polymers, as well as 1,4-butanediol in industries.
- Formaldehyde-based chemicals are also utilised in the production of vehicles and transmission as well as electrical system components.
Ketones Examples
Acetone
- Acetone is the most basic aliphatic ketone and a common organic solvent with industrial and chemical applications.
- To make a symmetrical ketone, a single carbonyl group is linked to two alkyl groups (CH3).
- It’s a colourless, fragrant, volatile, and viscous liquid found in plants, trees, car exhaust, fire, and fat metabolism.
- Acetone can also be found in low concentrations in urine and blood in healthy people, although it may be higher in diabetics.
- Because of its ability to dissolve a wide range of fats, resins, cellulose, ethers, and esters, acetone is frequently utilised in industry.
- Furthermore, it is employed as a chemical intermediary in pharmaceuticals as well as solvents for cosmetics and paintings.
- However, at large concentrations, it is harmful to living things, and many human activities such as fire, sewage, and urbanisation increase the concentration of acetone in the environment.
Aldehyde and Ketone Citations
- https://www.britannica.com/science/ketone
- https://www.engineeringtoolbox.com/acetone-2-propanone-dimethyl-ketone-properties-d_2036.html
- https://www.coursehero.com/file/p65of5r/quantities-in-urine-and-blood-larger-amounts-may-be-found-in-the-urine-and/
- https://www.chemguide.co.uk/organicprops/carbonyls/oxidation.html
- https://en.wikiquote.org/wiki/Aldehyde
- https://en.wikipedia.org/wiki/Organic_chemical_nomenclature
- https://en.m.wikipedia.org/wiki/Transmission_(mechanics)
- https://diabetestalk.net/ketosis/synthesis-of-ketones-from-carboxylic-acids
- https://www.encyclopedia.com/science-and-technology/chemistry/organic-chemistry/carbonyl-group
- https://www.britannica.com/science/aldehyde/Tautomerism
- https://www.britannica.com/science/aldehyde
- https://www.britannica.com/science/acetone
- https://www.brightstorm.com/science/chemistry/organic-chemistry/functional-groups/
- https://quizlet.com/99688524/chapter-1-organic-compounds-flash-cards/
- https://quizlet.com/130677478/ecology-chapter-2-flash-cards/
- https://celluloseether.com/
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example