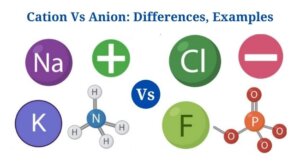
Definition of Cation
A cation is an atom or a group of atoms that has one or more positive electric charges.
- Cations are created in a variety of methods, including:
- When electrons from neutral atoms, ions, or other molecules are eliminated.
- When positive ions are combined with other molecules.
- When the shared pair of electrons is linked with one atom due to covalent rupture, the other atom becomes deficient.
- Cations are normally made up of metal atoms; however, positive radical ions, such as ammonium ion (NH4+), can have several atoms.
- Cations have more protons than electrons, hence they are positively charged. As a result, cations have an electron deficit.
- The ionic radius of cations is used to determine their size, as well as cations, on average, have a smaller radius than their parent atoms since they have one less orbit.
- Hydrogen is the smallest cation that doesn’t have an electron, making it significantly smaller than its parent atom.
- Anions occupy the majority of the space in the lattice of crystalline materials, and cations are consequently present between those spaces.
- Cations are highly reactive in the gaseous state and will react with anions to generate neutral molecules. Cations, on the other hand, can exist in both the liquid and solid states.
- When cation is liquid, it combines with the solvent to generate solvated ions, which are significantly more stable.
- Since these are charged particles, magnetic fields can deflect their movement.
- Cations travel towards the negative terminal (anode) of an electric field to produce neutral atoms. Most metals are refined using this method, which involves plating them onto an anode plate in an electric field.
- To represent the number of charges carried by these particles, cations are given distinct names. Dications have two positive charges, whereas trications have three positive charges.
- Similarly, carbocations are positively charged ions produced from organic compounds.
Definition of Anion
An anion is a molecule or a group of molecules with one or more negative electric charges.
- Anions can be created in a variety of ways, including:
- When electrons are added to neutral atoms, ions, or other compounds.
- When negative ions are combined with other molecules.
- When the shared pairing of electrons are linked with one atom due to covalent rupture, the consequence is a negative charge.
- Negative radical ions, such as the sulphate ion (SO4—), are commonly produced from non-metals; nevertheless, negative radical ions can have several atoms.
- Since anions have more electrons than neutrons, they are negatively charged. As a result, anions have a lot of electrons.
- The ionic radius is used to determine the size of ions, as well as anions, in general, have a bigger radius than their corresponding parent atoms since they contain more electrons repelling each other.
- Since anions are larger than solids, they take up the majority of the space in the crystal.
- Anions are very reactive in the gaseous state as well as will react with cations to generate neutral molecules. Anions, on the other hand, can exist in both the liquid as well as solid states.
- When an anion interacts with a solvent in a liquid state, it forms solvated ions, that are significantly more stable.
- Anions travel towards the positive terminal (cathode) in an electric field to generate neutral atoms.
- The majority of non-metallic gases are acquired by this method, that involves collecting them from an electric field’s positive terminal (cathode).
- The number of charges carried by anions is indicated by the names given to them. Anions with two negative charges are dianions, as well as anions with three negative charges are trianions.
- Carbanions are negative ions that are produced from organic compounds.
Difference between Cation and Anion
(Cation Vs Anion)
[ninja_tables id=”5437″]
Cation Examples
Sodium-ion is an example of a cation.
- The ionisation of sodium atom produces a monoatomic monocation, sodium atom.
- Sodium is a metal that gives the shared pair of electrons to the anion during bond breakage, resulting in a positive charge.
- The molecular formula of sodium ion is Na+, as well as the ionic radius is 0.102 nm.
- Sodium ions are required for a variety of physiological functions in the body, including the regulation of body fluids such as blood, nerve impulse transmission, heart activity, as well as other metabolic functions.
- Other creatures require sodium since it is maintained at a high concentration in their blood as well as other extracellular fluids, whereas plants do not require it.
- Humans need less than 500 mg of sodium each day in their diet.
- However, increased sodium intake may have a negative effect on health in persons with salt-sensitive blood pressure.
Anion example
An anion chloride ion is an example of an ion.
- The chloride ion is a diatomic monoanionic produced when the chlorine atom is ionised.
- Chlorine is a non-metal that takes the shared pair of electrons after bond rupturing, resulting in a negative charge.
- The chlorine ion has a chemical formula of Cl– as well as an ionic radius of 0.181 nm.
- The chloride ion is an electrolyte that is found in practically all bodily fluids. It is in charge of conveying nerve impulses, controlling fluid in as well as out of cells, as well as maintaining acid/base balance.
- The kidneys keep a close eye on the amount of chloride ions in the blood.
- Chloride-transporting proteins (CLC) are a type of protein that is found in a variety of organs, including the cell membrane as well as intracellular membranes.
- In mammals, CLC proteins belong to a gene family with nine members, at least four of that are involved in human genetic disorders.
Cation vs Anion Citations
- https://www.worldatlas.com/articles/what-is-the-difference-between-a-cation-and-an-anion.html
- https://www.emedicalprep.com/study-material/chemistry/solid-state/interstitial-sites-close-packing/
- https://www.britannica.com/science/ion-physics
- https://www.bbc.co.uk/bitesize/guides/ztc6w6f/revision/1
- https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-ion
- https://en.wikipedia.org/wiki/Anion
- https://answersdrive.com/what-does-chloride-do-for-water-4388216
- https://www.thoughtco.com/definition-of-anion-and-examples-604344
- https://www.enotes.com/homework-help/why-electrons-flow-from-cathode-anode-during-flow-686303
- https://sciencestruck.com/difference-between-cation-anion
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example