What is Ethanol Metabolism?
- The metabolization of alcohol involves a number of processes or pathways.
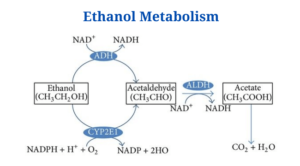
- One of most prevalent of these processes involves two enzymes: alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ADH) (ALDH).
- These enzymes help break down the alcohol molecule so that it may be eliminated from the body.
- ADH transforms alcohol to acetaldehyde, a highly toxic molecule which is also known to cause cancer.
- Acetaldehyde is then subdivided into acetate, a less active byproduct, which is then broken down into water and carbon dioxide for simple elimination in a second phase.
Ethanol Metabolism Location
Kidney and liver cells are two types of cells that can be found in the human body. The liver metabolises more than 80% of the ethanol that is ingested.
- Ethanol and two NAD+ are used as substrates.
- Acetate and two NADH2 are the end products.
Overview of pathway
- Alcohol dehydrogenase converts ethanol to acetaldehyde, a process that requires one NAD+.
- Acetaldehyde is an unstable molecule that is prone to generating free radical structures, causing damage to adjacent tissues. Acetaldehyde dehydrogenase then oxidises it to acetate.
- One NAD+ is required for this reaction once again.
- Acetate can be eliminated in the urine (which happens most of the time) or converted into acetyl CoA and introduced into the citric acid cycle.
The liver microsomal ethanol oxidising system (MEOS), which requires a cytochrome P450–containing enzyme, is the alternative route of metabolism.
Important Enzymes
Alcohol dehydrogenase: The reaction is catalysed by NAD + and zinc, and it follows zero-order kinetics.
Acetaldehyde dehydrogenase: NAD + is required for its activity; disulfiram, a medication used to treat alcoholism, inhibits it.
Related Diseases
- Acetaldehyde is an unstable chemical that can produce free radicals, which can be harmful to the liver (leading to cirrhosis).
- Acetaldehyde is hypothesised to be implicated in the neurologic signs of foetal alcohol syndrome and can be harmful to embryological neural crest tissue.
- When alcoholics consume ethanol, they risk developing hypoglycemia.
- Because of the higher ratio of NADH/NAD+ caused by ethanol metabolism, pyruvate and oxaloacetate are converted to lactate and malate, respectively.
- Because pyruvate and oxaloacetate are intermediates in gluconeogenesis, gluconeogenesis is slowed, which can lead to hypoglycemia.
Ethanol Metabolism Citations
- David Hames and Nigel Hooper (2005). Biochemistry. Third ed. Taylor & Francis Group: New York.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results