Flow Cytometry – What is it?
- For the detection and measurement of physical and chemical properties of cells or particles in heterogeneous fluid mixtures, flow cytometry is commonly used with laser-based technology.
- This technique has gained in popularity over time since it provides for a fast examination of various cell characteristics (including qualitative and quantitative).
- By utilising this technology, it is possible to determine a particle’s size, texture or internal complexity, and fluorescence intensity.
- An optical-to-electronic coupling device recognises cells based on lasers distributed by them in order to quantify such qualities
- Though its name implies it only deals with cells, it can also deal with chromosomes and molecules as well as a range of particles that can be suspended in fluid.
Flow Cytometry Principle
If you’re unfamiliar with flow cytometry, it is based on the idea that particles scatter light and fluorescence when they are passed by a laser beam.
Light Scattering
- Light scattering happens when a particle reflects incident laser light. How much this happens depends on a particle’s physical properties, such as its size and internal complexity, but it’s not impossible.
- Cell surface area or cell size affects the amount of forward-scattered light (FSC). Photodiodes disperse incident laser light in a forward direction, detecting rays that are just off axis.
- This is a measurement of mostly diffracted light. Cellular complexity and granularity are shown by side-scattered light (SSC).
- At every cell contact where the refractive index varies, SSC is used to measure the amount of refracted and reflected light that passes through the cell. Cell types in a heterogeneous cell population can be differentiated using FSC and SSC measurements.
Fluorescence
- To monitor the expression of biological entities, such as proteins and nucleic acids, fluorescent markers are used.
- Radiation energy from the fluorescent substance absorbs light in a specific wavelength range.
- As a result of the light absorption, the fluorescent compound’s electron is boosted to a higher energy level.
- Excitement is released as a fluorescence, which is detected by detectors, and the electron swiftly returns to its ground state, releasing the surplus energy.
- Diverse fluorochromes can be utilised to differentiate different subpopulations of cells in a mixed population.
- For each subpopulation, the fluorescence pattern of each subpopulation can be used to determine which cells are present in a sample, and to count their relative percentages.
- Following this process, an electronic system turns the detected light into electronic signals that are then analysed by the computer.
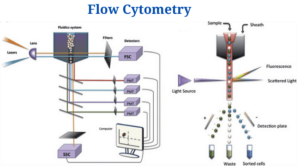
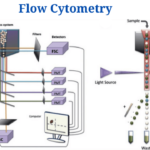
Instrumentation and Parts of Flow Cytometry
Flow cytometers consist of three basic systems: fluidics, optics, and electronics.
Fluidics
- To transport particles through a fluid stream, the fluidics system is used. An injection of sheath fluid (usually buffered sodium chloride solution) into the flow chamber is required to achieve this.
- There’s no way around that. The sheath fluid’s centre of gravity is right where you want your sample to be in order to have a laser beam interact with it.
- To focus the sample suspension, it is injected into the centre of a sheath liquid stream. Fluid in the sheath moves the particles about and confines them to the centre of the sample core.
Optics System
- The cytometer’s optical system is made up of excitation optics and collecting optics.
- A laser and lenses used to shape and focus it on a flow of a sample are called excitation optics.
- These optics consist of an interferometer, a collecting lens, and an optical mirror system that diverts the collected light at defined wavelengths to designated optical sensors.
- It is possible to gather the signals from a cell or particle that has passed through the laser light by using the side rays and fluorescence signals that are directed to the photomultiplier tubes (PMTs).
- In order to increase the specificity of a detector for a particular fluorescent dye, the tubes are covered with a filter. As a result, only a limited range of wavelengths may be detected by the detector.
Electronics System
- As a result of this, the detector signals are converted into digital signals that a computer can interpret.
- An amplifier converts an electrical current into a voltage pulse when light strikes one side of the PMT or photodiode; the electrons are amplified to create a higher quantity of electrical current.
- An Analog-to-Digital Converter (ADC) converts the pulse from an analogue number when it reaches its highest peak when the particle strikes the centre of the beam (the scattering or fluorescence is at its maximum) (ADC).
Flow Cytometry Protocols, Procedures, Processes, and Steps
Flow cytometry is made up of the following steps:
Preparation of the Sample
- The cells under investigation must be in a single-cell suspension before being put through the flow cytometers.
- Before examination, clumped cultured cells or cells found in solid organs should be transformed into a single cell suspension by enzymatic digestion or mechanical dissociation of the tissue, as appropriate.
- It is then followed by mechanical filtering in order to eliminate instrument blockages and provide better flow data.
- The cells are then treated with unlabeled or fluorescently conjugated antibodies in test tubes or microtiter plates and evaluated using a flow cytometer system.
Staining with Antibodies
- The cells are coated with fluorochrome-conjugated antibodies specific for the surface markers present on various cells once the sample has been produced. Direct, indirect, or intracellular staining can be used to accomplish this.
- In indirect staining, cells are treated with a fluorophore–conjugated antibody.
- The fluorophore-conjugated secondary antibody recognises the primary antibody in indirect staining.
- Within cells, antigens can be measured directly using intracellular staining procedures. They must first be permeabilized before being stained with antibodies in the permeabilization buffer.
Running Samples
- It is first necessary to adjust the voltages in each sensor by running control samples first.
- In the cytometer, the flow rates have been established, and the sample has been run through the cytometer.
Types of Flow Cytometry
Because of this, flow cytometers come in varied shapes and sizes.
- Traditional Flow cytometers
- They use sheath fluid to focus the sample stream, which is the most popular type of cytometer nowadays.
- These lasers are employed in flow cytometers: 488 nm (blue), 405, 520, 561, 640, 355nm, and 355 nm (green-yellow, green, and red) (ultraviolet).
- Acoustic focus Cytometers
- Ultrasonic waves are utilised in these cytometers to focus cells for examination.
- In addition, this stops sample clogging and permits for higher sample inputs to be used.
- Cell sorters
- After processing, cell sorters are a type of classic flow cytometer that permits the user to collect the samples following they have been processed.
- To do this, use a cell separator to segregate the cells that have a positive parameter from those who have a negative value.
- Imaging Flow cytometer
- Imaging cytometers are cytometers that integrate fluorescence microscopy with regular cytometers.
- Single cell and population-level fluorescence measurements can be made quickly with an imaging-cytometer by measuring morphology and several fluorescence parameters.
Applications/Uses of Flow cytometer
There are several applications for flow cytometry, such as molecular biology, pathology and immunology as well as plant and marine biology. These are some of the most prevalent applications:
- Leukemia and other malignancies can be detected with this technique in clinical laboratories.
- This can be done by using cytometers such as cell sorters to physically separate the cells of interest.
- Using fluorescent markers, it may be used to detect DNA content.
- As a result of utilising fluorescent dye for four separate stages of the cell cycle, flow cytometers can analyse replicating cells.
- For the study of multi-drug resistant bacteria in the blood and other samples, acoustic flow cytometers are utilised.
- According to the differences in morphological and biochemical changes, flow cytometers can detect the different stages of cell death (apoptosis and necrosis).
Limitations of Flow cytometer
- Because of the way it works, we can’t get any information about the intracellular location or distribution of proteins.
- In the long run, debris accumulates, which might lead to erroneous results.
- Staining and sample preparation require a lot of pre-treatment, which takes a long time.
- Costly and labor-intensive, flow cytometry is only feasible with highly qualified workers.
Flow cytometer Citations
- McKinnon K. M. (2018). Flow Cytometry: An Overview. Current protocols in immunology, 120, 5.1.1–5.1.11. https://doi.org/10.1002/cpim.40
- Dean, P.N. and Hoffman, R.A. (2007), Overview of Flow Cytometry Instrumentation. Current Protocols in Cytometry, 39: 1.1.1-1.1.8. DOI:1002/0471142956.cy0101s39
- https://enquirebio.com/flow-cytometry
- – https://www.sciencedirect.com/science/article/pii/S0169409X04001838
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4856776/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC100149/
- https://www.nature.com/articles/s41467-020-14929-2
- https://www.labome.com/method/Flow-Cytometry-A-Survey-and-the-Basics.html
- https://www.bdbiosciences.com/en-us/instruments/research-instruments/research-cell-sorters/facsaria-iii
- https://answers.yahoo.com/question/index?qid=20120419080608AAzfZea
- http://sgpwe.izt.uam.mx/files/users/uami/cobe/Adan-Flow_cytometry-basic_principles_and_applications.pdf
Related Posts
- Anisocytosis: Definition, Types, Causes, Symptoms, Treatment
- Endospore Staining: Principle, Procedure, Reagents, Results
- Flow Cytometry: Overview, Principle, Steps, Types, Uses
- Northern Blot: Overview, Principle, Procedure and Results
- MPV Blood Test: Calculation, High and Low MPV Value, Results
- Latex Agglutination Test: Objectives, Principle, Procedure, Results
- Iodine Test: Definition, Objective, Principle, Procedure, Results
- Eosin Methylene Blue (EMB) Agar
- Biuret Test for Protein: Purpose, Objectives, Principle, Procedure, Reagents
- Streak Plate Method: Meaning, Principle, Methods, Importance, Limitations
- Bile Solubility Test: Objective, Principle, Procedure, Results, Uses
- Butyrate Disk Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Beta Lactamase Test: Objective, Principle, Procedure, Results, Limitations
- Bacitracin Susceptibility Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Bile Esculin Test: Objective, Principle, Procedure, Result, Uses, Limitations