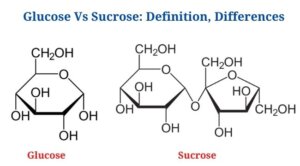
Definition of Glucose (What is Glucose?)
Glucose is a basic sugar that serves as the principal energy source in living systems.
- Glucose is the most abundant monosaccharide on the planet, as well as it is found as well as used by a wide range of organisms.
- The principal sources of glucose on Earth are green plants as well as algae, which produce glucose through the photosynthesis process, which involves the use of carbon dioxide as well as water.
- Living organisms consume monomeric glucose for immediate energy, but it can also be stored as a polymer for later use.
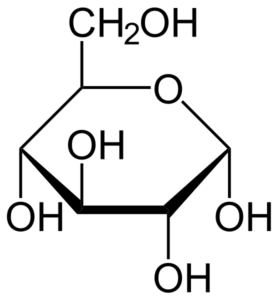
- Glucose is an aldohexose, which is a type of monosaccharide made up of six carbon atoms as well as an aldehyde functional group.
- The word glucose comes from the French word glukos, which means sweet, as well as refers to the molecule’s sweet taste.
- Glucose molecules can have an open-chain or acyclic structure, as well as a ring or cyclic structure.
- Glucose comes in two enantiomeric forms: D-glucose as well as L–glucose. D-glucose, commonly known as dextrose, is abundant in nature, but the L-glucose is uncommon.
- Since glucose is a monosaccharide, it can be made from the hydrolysis of various polymeric sugars such as lactose as well as sucrose.
- Glucose has the chemical formula C6H12O6or H-(C=O)-(CHOH)5-H, with the five hydroxyl groups organized in a precise pattern along the six-carbon backbone.
- Glucose is the most extensively employed aldohexose in most living species, which can be explained by the molecule’s lesser proclivity for reacting nonspecifically with proteins’ amine groups.
- When compared to other aldohexoses, glucose can persist in a more stable cyclic state, which results in lesser reactivity towards proteins.
- In living systems, glucose is frequently found as polymers rather than in its free state in order to conserve energy.
Definition of Sucrose (What is Sucrose?)
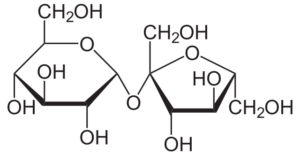
Sucrose is a disaccharide that is made up of two monosaccharides, fructose as well as glucose, in a 1:1 ratio.
- Sucrose is derived in vast quantities naturally from plants such as sugarcane as well as maple, as well as the majority of it is consumed as food.
- Since sucrose may be broken down into smaller, simpler units, it is chemically more complicated than glucose.
- The term sucrose is derived from the French word sucre, which means sugar, signifying the compound’s source as well as nature.
- In sucrose, the monomeric units are joined by an ether bond between the C1 on the glucose as well as the C2 on the fructose.
- Unlike other disaccharide connections, the glycosidic bond in sucrose is generated between the reducing ends of the monomers.
- The glycosidic link in sucrose prevents sucrose from reacting with cellular macromolecules by inhibiting monomer-to-monomer interaction.
- Hydrolysis is the process of breaking down sucrose to liberate monomeric units, although sucrose hydrolysis is incredibly slow, since a sucrose solution can go for years without hydrolysis.
- However, introducing sucrase enzymes can speed up the hydrolysis process. The addition of acid, which destroys the acetal link between the monomers, can also cause sucrose to hydrolyze.
- Sucrose, like fructose, is the end result of photosynthesis as well as thus can be found naturally in a variety of plants.
- In other living things, sucrose is used as an intermediary in several metabolic pathways rather than as a store sugar.
- Since the reducing ends of the monomers in sucrose are engaged in the creation of the glycosidic bond, sucrose is a non-reducing sugar.
Key Differences between Glucose and Sucrose
(Glucose Vs Sucrose)
[ninja_tables id=”5634″]
Glucose Vs Sucrose Citations
- https://wiki2.org/en/Sucrose
- https://quizlet.com/244811195/carbs-tbich-flash-cards/
- https://www.reference.com/science/takes-place-during-hydrolysis-sucrose-a0bba9c79f1ce9cf
- https://www.ausetute.com.au/redsugar.html
- https://www.answerout.com/sucrose-is-a-disaccharide-composed-of-the-monosaccharides-glucose-and-fructose-the-hydrolysis-of-sucrose-by-the-enzyme-sucrase-results-in-2/
- https://diabetestalk.net/blood-sugar/difference-between-glucose-and-fructose-structure
- https://diabetestalk.net/blood-sugar/difference-between-glucose-and-sucrose-chemically
- https://www.reference.com/science/end-product-photosynthesis-a76e7b5e92a3d21b
- https://www.answers.com/Q/Glycosidic_linkage_between_glucose_and_fructose
- https://pediaa.com/what-is-the-difference-between-blood-sugar-and-glucose/
- https://en.wikipedia.org/wiki/Sugar
- https://diabetestalk.net/blood-sugar/what-is-the-difference-between-the-structure-of-glucose-and-fructose
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example