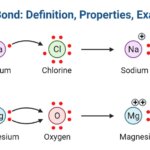
Definition of Hydrogen Bond
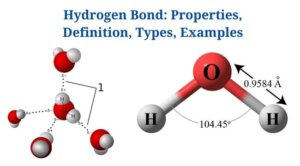
A hydrogen bond is an attraction between a molecule’s hydrogen atom as well as more electronegative atoms of the same molecule or other molecules.
- Since a hydrogen bond is weaker than a covalent bond, it has a greater bond length. A dotted line (…) denotes the hydrogen bond.
- Fluorine, nitrogen, as well as oxygen are electronegative elements that are commonly engaged in hydrogen bonding. Hydrogen bonds with sulfur, chlorine, as well as carbon have also been reported, though they are weaker.
- A hydrogen bond is a sort of polar covalent bond in which the pair of electrons between hydrogen as well as another atom is unequally distributed, resulting in modest charges on both atoms.
- It’s been suggested that the bond has some ionic components as a result of partially charged ions forming.
- Although the hydrogen bond is an electrical dipole-dipole contact, it is achieved in order for both atoms to have a stable electronic state.
- Hydrogen bonding is an important interaction since it gives the compounds created varied characteristics. Furthermore, since a hydrogen bond can form between two molecules, it plays a role in the structure as well as function of the resulting compounds.
- It’s a weaker bond than an ionic or covalent bond, but it’s more powerful than Van der Waal’s force.
- Hydrogen bonding takes place in both organic as well as inorganic compounds, but only when the carbon atoms are bonded to a more electronegative atom in organic molecules.
Hydrogen Bonding Conditions
The prerequisites for the development of hydrogen bonds between chemical compounds are as follows:
- One of the two atoms should be hydrogen, since it is highly electropositive as well as tiny in size, increasing the bond’s polarity.
- To induce a dipole moment, the other atoms bonded to hydrogen should be highly electronegative.
- Electronegative atoms should be modest in size since smaller atoms have a stronger electrostatic attraction.
- In the case of intermolecular hydrogen bonding, the electronegative atom of the other molecule must be bonded to a hydrogen atom.
Properties of Hydrogen Bonds
The physical properties of the compounds change as a result of hydrogen bonding. Here are a few examples:
1. Boiling and melting point
- Hydrogen bonds have an impact on a compound’s boiling as well as melting point since a stronger hydrogen bond results in more intermolecular attraction.
- As a result, it takes a lot of heat energy to break down the forces as well as cause melting or boiling.
- Intermolecular hydrogen bonding causes molecules in a compound to exist as linked molecules.
- The association raises the compound’s size as well as molecular mass, resulting in a higher Van der Waal’s force.
- As a result, compounds with a greater number of hydrogen bonds have a higher boiling point as well as melting point.
2. Surface tension and density
- Other physical properties such as density, surface tension, and even viscosity are affected by hydrogen bonding.
- The compounds have more connections or linkages between molecules, resulting in higher compound density.
- Since the molecules on the surface are connected to one another via these bonds, the surface tension increased.
3. Physical State
- Hydrogen-bonded compounds can exist in either a liquid or a gaseous state. The liquid form exists for compounds with stronger bonds, while the gaseous state exists for compounds with weaker hydrogen bonds.
- The electronegativity of the atom linked to hydrogen determines the bond strength. Hydrogen bonds in H2O are stronger since oxygen has a higher electronegativity, whereas hydrogen bonds in H2S are weaker since sulfur is less electronegative than oxygen.
4. Molecular associations
- As a result of a weak interaction between molecules, compounds containing hydrogen bonds form aggregates.
- Hydrogen bonding causes the development of dimers as well as trimers in many substances.
5. Solubility
- Since hydrogen bonding is involved in the hydration of anions in aqueous solutions, it is responsible for the solubility of many compounds.
- Hydration contributes to the compounds’ solvent properties as well as aids in the dissolution of certain ionic compounds.
Types of Hydrogen Bond
Hydrogen bonds can be classified into two types depending on their occurence
1 . Intra-molecular hydrogen bonds
- The intramolecular hydrogen bond is the bond produced within a molecule between hydrogen atoms as well as electronegative atoms such as nitrogen, oxygen, as well as sulfur.
- Hydrogen as well as an electronegative atom must be present in close proximity in order for an intramolecular hydrogen bond to form.
- Even if the atoms in some molecules, such as ethylene glycol, are widely apart, the molecular geometry of the compounds allows them to engage in hydrogen bonding.
- Intramolecular hydrogen bonding in a molecule has a significant impact on a molecule’s molecular structure as well as characteristics.
- The bonding, on the other hand, has little impact on the compound’s overall physical qualities.
2. Inter-molecular hydrogen bonds
- Intermolecular hydrogen bonding is a type of hydrogen bonding that takes place between two molecules that are either the same or different.
- As long as hydrogen as well as electronegative atoms are present, this sort of bonding can occur between any number of the same or different molecules.
- Intermolecular hydrogen bonding happens when two molecules are in close proximity to one another, just like intramolecular hydrogen bonding.
- Intermolecular hydrogen causes compounds to have various characteristics, such as enhanced surface tension as well as viscosity. These shifts are frequently dramatic.
How do hydrogen bonds form?
- Hydrogen bonds are produced when a hydrogen atom interacts with electronegative atoms such as nitrogen, oxygen, as well as fluorine.
- To achieve a stable electrical configuration, both hydrogen as well as the electronegative atom share one electron in the bond.
- Due to increased electron affinity, the common pair of electrons in the bond is pushed towards the more electronegative atoms.
- The production of charge in the atoms is caused by an unequal distribution of electrons between the atoms.
- Since the electronegative atom has a relatively high electron density, it creates a negative charge, whereas the hydrogen atom develops a positive charge due to its low electron density.
- Since the charge created on the atoms is less than a whole unit charge, they are referred to as delta positive (δ+) as well as delta negative (δ-).
- When atoms become polar, they develop a dipole moment, allowing them to interact with one another as well as other comparable atoms.
- The hydrogen bond is formed by the electrostatic force or dipole-dipole interaction between charged atoms.
- Intermolecular hydrogen bonding takes place when a slightly positively charged hydrogen atom interacts with a slightly negatively charged electronegative atom from another molecule.
- This form of contact serves as a link between molecules of the same or distinct compounds, allowing them to bond together.
- The strength of the hydrogen bond is determined by the degree of charge formed on the atoms, that is determined by the extent of shared electron displacement.
- As a result, the electronegativity of the atom involved in the bond formation determines the strength of the hydrogen bond within a molecule or between molecules.
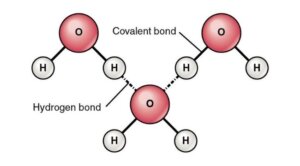
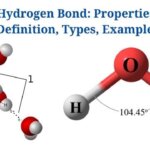
Hydrogen Bonding in Water
Figure: Hydrogen Bonding between Water Molecules. Image Source: Openstax
- In water, hydrogen bonds are the attractive forces that hold the compound’s molecular mass together.
- Hydrogen bonding takes place in both the liquid as well as solid ice states, where intermolecular as well as intramolecular interactions hold the molecules together.
- Within the water molecule, there are two hydrogen bonds, each with a dipole moment of 1.5D.
- Hydrogen bonding in water is responsible for water’s various physical properties. Water has a greater boiling point as well as melting point than other hydrogen compounds due to the creation of intermolecular hydrogen bonds.
- Since of hydrogen bonding, water can exist in the solid state as crystals when it freezes.
- Water can exist in the liquid state throughout a wide temperature range due to significant hydrogen bonding between its molecules.
- Hydrogen bonding is also responsible for water’s universal solvent feature. Water atoms quickly establish hydrogen bonds with electronegative atoms in various solutes, allowing for their solubility.
- Water’s hydrogen bonding also allows anions in an aqueous solution to be hydrated, enhancing water’s capacity to operate as a suitable ionic compound solvent.
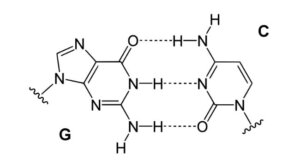
Hydrogen Bonding in DNA
Figure: Hydrogen bonding in DNA. Hydrogen bond between Guanine and Cytosine, one of two types of base pairs in DNA.
Image Source: Yikrazuul.
- Hydrogen bonds are required for the structure as well as function of nucleic acids, including DNA.
- The hydrogen bond is essential for DNA redundancy, transcription, as well as translation.
- Hydrogen bonds in base pairs, such as adenine in one strand as well as thymine in the other, connect nucleotide bases in DNA. Complementary nucleotides are held together in a helix by these connections.
- Each of the four bases has the ability to make hydrogen bonds with molecules in the outside world.
- Although the internal as well as external hydrogen bonds in DNA are weak, the molecule is stable due to the huge number of bonds in the molecule.
- The hydrogen bonds between bases also keep the bases in the double helix’s interior.
- Varying base pairings have different numbers of hydrogen bonds, with cytosine as well as guanine having three hydrogen bonds as well as adenine as well as thymine having two hydrogen bonds.
- The DNA double helix is similarly stabilized by hydrogen bonding across the helix axis, but not in the direction of the axis.
- The phosphate as well as sugar atoms in the deoxyribose-phosphate backbone operate as hydrogen bond acceptors. The DNA double helix is stabilized across the axis thanks to this hydrogen bond.
Hydrogen Bonding Examples
1 . Protein Hydrogen Bonding
- Proteins have hydrogen bonds in their secondary structure that hold the backbone oxygens together with amide hydrogens.
- Proteins’ α-helix secondary structure is generated by the regular spacing of amino acid residues between positions I as well as i+4.
- The presence of hydrogen bonding alternating residues on each participating strand forms the beta-sheet structure.
- Alkyl groups engage with some hydrogen bonds, resulting in the tertiary structure of proteins.
- Hydrogen bonds are important for the stability of multimeric proteins since they connect the subunits.
- Protein folding is influenced by hydrogen bonding, that affects the quaternary structure of some proteins.
2. Hydrogen bonding in NH3 molecule
- Ammonia molecules include three hydrogen bonds that are sustained by nitrogen’s electronegativity as well as its short size.
- However, since the electron comprises a single lone pair of electrons, the number of hydrogen bonds in ammonia is limited.
- Water has a stronger hydrogen bond than ammonia since oxygen has a higher electronegativity than nitrogen.
- Each hydrogen bond in ammonia has a 1.5D dipole moment that affects the molecule’s structure.
- Molecules could form intermolecular hydrogen bonds with other ammonia molecules or molecules from other substances.
Applications of Hydrogen Bonds
Hydrogen Bonds are used in a variety of ways.
- Distinct hydrogen-bonded organic as well as inorganic compounds have different applications in different fields.
- The structure as well as function of nucleic acids such as DNA as well as RNA are dependent on hydrogen bonding. The base pairing is sustained by varying numbers of hydrogen bonds in both RNA as well as DNA.
- Hydrogen bonding, either within the protein molecule or with external molecules, stabilize various protein configurations.
- Different features of water, such as high boiling point, surface tension, as well as solvent nature, are due to hydrogen bonding.
- Since most oral medicines include roughly 8-10 hydrogen bonds, hydrogen bonds play an important role in drug development.
Hydrogen Bond Citations
- Jeffrey G.A., Saenger W. (1994) The Role of Hydrogen Bonding in the Structure and Function of the Nucleic Acids. In: Hydrogen Bonding in Biological Structures. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-85135-3_20
- https://www.sciencedirect.com/topics/physics-and-astronomy/hydrogen-bonds
- https://www.britannica.com/science/chemical-bonding/The-hydrogen-bond
- https://quizlet.com/99507662/chapter-4-homework-flash-cards/
- https://qsstudy.com/chemistry/effects-hydrogen-bond-properties-compounds
- https://en.glosbe.com/en/en/intramolecular%20hydrogen%20bond
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_(Miessler_Fischer_Tarr)/03%3A_Simple_Bonding_Theory/3.04%3A_Hydrogen_Bonding
- https://byjus.com/jee/hydrogen-bonding/
- https://www.youtube.com/watch?v=1vm3od_UmFg
- https://www.thoughtco.com/what-causes-hydrogen-bonding-603991
- https://www.slideshare.net/RamyaAS2/protein-94237386
- https://www.sciencedirect.com/topics/chemistry/solubility
- https://www.priyamstudycentre.com/2020/03/hydrogen-bonding.html
- https://www.differencebetween.com/difference-between-intermolecular-and-vs-intramolecular-hydrogen-bonding/
- https://www.chemistrylearner.com/chemical-bonds/hydrogen-bond
- https://www.britannica.com/science/water/Structures-of-ice
- https://www.brainkart.com/article/Importance–Strength–Types-of-Hydrogen-bonds—Intermolecular-Forces_2808/
- https://pediaa.com/how-do-polar-and-nonpolar-molecules-interact-with-each-other/
- https://en.wikipedia.org/wiki/Hydrogen-bonding
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example