What is MUG Test?
MUG Test: MUG stands for 4-Methylumbelliferyl-β-D Glucuronide and it acts as a substrate for the organisms those having enzyme β-glucuronidase. This enzyme is present in 97% strains of E. coli .Organisms other than E. coli of the Enterobacteriaceae family Salmonella, Shigella and Yersinia also possess the enzyme β-glucuronidase.
Objectives of MUG test
To identify a variety of Enterobacteriaceae genera and Escherichia coli strains that produce verotoxin.
Principle of MUG test
β-D-Glucuronidase, an enzyme generated by most strains of E. coli and other Enterobacteriaceae, hydrolyzes β-d-glucopyranosid-uronic derivatives to aglycons and d-glucuronic acid. The disc is impregnated with the substrate 4-methylumbelliferyl-beta-D-glucuronide. A fluorogenic substrate of β -glucuronidase is 4 -methylumbelliferyl-beta-D-glucuronide which is abbreviated as MUG. The organism that generates the – D- Glucuronidase enzyme may break the substrate 4- Methylumbelliferyl— D- Glucuronide, resulting in the fluorescent moiety 4-methylumbelliferyl (4-MU). Under long wavelength UV light, the final product of hydrolysis, 4-methylumbelliferyl (4-MU), fluoresces blue. MUG is not produced by verotoxin-producing E. coli strains. Hence a negative test result could suggest the presence of a clinically significant strain. At low (1.97-6.72) and high (7.12-10.3) pH, 4–MU fluorescence has excitation maxima of 320 and 360 nm, respectively.
Procedure of MUG test
Direct Disk Test
- Add a drop of demineralized water to a MUG disc which is kept in an empty sterile petri dish.
- On the disc, smear 2-3 isolated colonies from an 18-24 hour old culture.
- To create a humid environment, place a piece of filter paper saturated with water in the lid of the petri dish.
- At 35°-37°C, incubate aerobically for up to 30 minutes.
- After incubation, inspect the disc for fluorescence in a darkened area using longwave UV light (360nm).
Tube Test
- In a clean glass tube, pour 0.25ml of demineralized water.
- In the tube, make a heavy suspension containing 3- 4 colonies of test isolate.
- Place a MUG disc in the tube with forceps and shake vigorously to ensure enough substrate elution in the surrounding liquid.
- Incubate aerobically for up to 1 hour at 35°-37°C.
- After incubation, inspect the disc for fluorescence in a darkened area using longwave UV light (360nm).
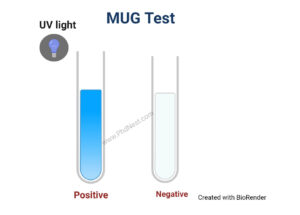
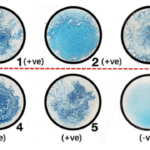
Result of MUG Test
Positive test: blue fluorescence
Negative test: no fluorescence
Uses of MUG Test
- The test is used to presumptively identify the Enterobacteriaceae genera.
- To identify Escherichia coli strains that produce verotoxin. (Strains of E. coli that produce verotoxin do not produce MUG. Hence a negative test result could indicate the presence of a clinically significant strain.)
- Escherichia coli can be detected in water and food samples with this tool.
Limitations of MUG Test
- This test is merely one element of a larger method for detecting E. coli. For conclusive identification, additional biochemical and serological testing is required.
- The majority of E. coli 0157:H7 strains are MUG negative. For quick identification of isolates during outbreaks, an E. coli 0157:H7 latex agglutination test has to be used in combination with the MUG test.
- MUG-positive Shigella strains exist. To distinguish Shigella and E. coli, serological tests may be required.
- When test colonies isolated from media-containing dyes (EMB, MAC) are utilized, false-negative results may be reported, making interpretation problematic.
- Salmonella, Shigella, Staphylococcus, and Streptococcus are examples of organisms that have the enzyme -Glucuronidase and are MUG positive. Only lactose-positive, gramme negative rods are tested for – Glucuronidase activity, which helps prevent other organisms from being misidentified as E. coli.
Quality control of MUG test
Positive: Escherichia coli (ATCC25922)
Negative: Klebsiella pneumoniae (ATC13883)
Mug Test Citations
- Remel MUG disk. 2010. 12076 Santa Fe Drive, Lenexa, KS 66215, USA https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU21135.pdf
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/MUGDisks.html
- https://eportal.mountsinai.ca/Microbiology//manual/tech/tech25.pdf
- himedialabs.com/TD/M1461.pdf
- https://www.atcc.org/en/Global/FAQs/D/4/E_coli_and_the_MUG_test-816.aspx
- https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU21135.pdf
Related Posts
- Anisocytosis: Definition, Types, Causes, Symptoms, Treatment
- Endospore Staining: Principle, Procedure, Reagents, Results
- Flow Cytometry: Overview, Principle, Steps, Types, Uses
- Northern Blot: Overview, Principle, Procedure and Results
- MPV Blood Test: Calculation, High and Low MPV Value, Results
- Latex Agglutination Test: Objectives, Principle, Procedure, Results
- Iodine Test: Definition, Objective, Principle, Procedure, Results
- Eosin Methylene Blue (EMB) Agar
- Biuret Test for Protein: Purpose, Objectives, Principle, Procedure, Reagents
- Streak Plate Method: Meaning, Principle, Methods, Importance, Limitations
- Bile Solubility Test: Objective, Principle, Procedure, Results, Uses
- Butyrate Disk Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Beta Lactamase Test: Objective, Principle, Procedure, Results, Limitations
- Bacitracin Susceptibility Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Bile Esculin Test: Objective, Principle, Procedure, Result, Uses, Limitations