Polar Molecules Definition
A polar molecule is a chemical compound with an unequal electron distribution between the atoms, resulting in a dipole moment.
- Polarity refers to the difference between a molecule’s electrical poles, that indicates how polar the molecule is.
- The sum of all the bonds in a molecule determines whether or not the molecule is polar.
- When a bond is formed between atoms with different electronegativities, polarity develops. When one of the atoms has a stronger electron affinity, the shared pair of electrons are drawn to it, changing the electron distribution.
- A polar bond will develop if the difference in electronegativity is significant.
- Polarity is commonly expressed in the case of covalent bonds, as these bonds are established by the sharing of electrons. If the difference is great, one of the atoms totally strips the electrons towards it, resulting in an ionic bond.
- In the presence of an electric field, polar molecules orient themselves in a specific orientation. The positive end of the molecules points to the field’s negative pole, whereas the negative end points to the positive pole.
- When determining the polarity of molecules with more than two atoms, the geometry of the molecules should be taken into account.
- Polarity in polyatomic compounds is determined by the net dipole moment of all covalent bonds in the molecule.
- Since polar connections are much stronger than nonpolar links, polar compounds usually have a higher boiling as well as melting temperature, as well as a high surface tension.
- H2O, CHF3, NH3, as well as other polar compounds are examples.
Nonpolar Molecules Definition
Nonpolar molecules are those in that the electrons are equally shared among the involved atoms as well as have a zero dipole moment.
- Nonpolar molecules are created when the two atoms in a chemical bond have a similar affinity for electrons, allowing for equal electron distribution between them.
- Only amongst diatomic molecules of the same element, such as N2 and O2, do true nonpolar bonds exist.
- Nonpolar molecules, on the other hand, display London dispersion forces caused by the symmetrical distribution of electrons.
- The interaction is generally random and transient, and it is the weakest of all intermolecular forces.
- The lack of polarity in polyatomic or complex molecules can be caused by the symmetrical arrangement of polar bonds.
- A zero net dipole can be generated by arranging two equally polar bonds in such a way that the dipole of one bond cancels out the dipole of the other, resulting in a nonpolar molecule.
- Because the gap in electronegativity between carbon and hydrogen atoms is so minimal, most hydrocarbons are nonpolar.
- Because there is no electrostatic link, nonpolar interactions are usually weaker than polar contacts.
- Nonpolar molecules have no orientation in an electric field because there are no electrostatic interactions between nonpolar molecules and the electric field.
- Nonpolar compounds include CO2, H2, benzene, and others.
Differences between Polar and Nonpolar Molecules
(Polar and Nonpolar Molecules)
[ninja_tables id=”5442″]
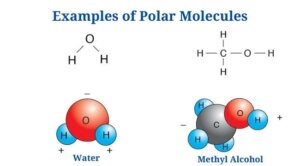
Polar Molecules Examples
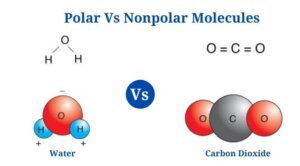
Water (H2O)
- Water is a polar molecule made up of two O-H bonds, each with a dipole moment of 1.5D.
- Water lacks a linear structure since the oxygen atom’s lone pair of electrons repels the shared pair of electrons between hydrogen as well as oxygen.
- Since the oxygen atom has a higher electronegative charge than hydrogen, it attracts the shared pair of electrons to itself.
- Since of the unequal distribution of electrons, the oxygen atoms have a partial negative charge as well as the hydrogen atoms have a partial positive charge. The bending structure of the molecule is due to the polarity of the O-H bonds.
- Since hydrogen molecules are polar, they can establish hydrogen bonds with other polar molecules or with other water molecules.
- Water is known as a universal solvent since of the interaction between its slightly charged atoms, that allows it to dissolve most polar compounds.
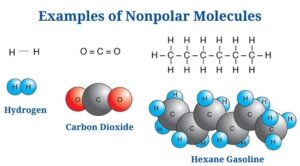
Nonpolar Molecule Examples
Carbon dioxide is an example of a nonpolar molecule (CO2)
- Carbon dioxide is made up of two polar C-O bonds, but the molecule is nonpolar since the links are organized so that the net dipole is zero.
- The dipole moment of each C-O bond is 2.3D, but the dipoles act in opposite directions, resulting in a net-zero dipole.
- The C-O bonds are organized at an angle of 180 degrees to one another, resulting in a molecular structure that is linear.
- The C-O polarity is caused by the oxygen atom being more electronegative than the carbon atom.
- Even though the bonds in the molecule are polar, carbon dioxide is an example of a nonpolar molecule.
Polar Vs Nonpolar Molecules Citations
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- https://chem.libretexts.org/Courses/Brevard_College/CHE_103_Principles_of_Chemistry_I/05%3A_Chemical_Bond_II/5.10%3A_Electronegativity_and_Bond_Polarity
- https://www.vedantu.com/chemistry/polarity
- https://pediaa.com/difference-between-polar-and-nonpolar-molecules/
- https://techiescientist.com/is-pf5-polar-or-nonpolar/
- https://www.thoughtco.com/definition-of-polar-molecule-605531
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example