Definition of Saturated Hydrocarbons
Saturated hydrocarbons are the most basic types of hydrocarbons, consisting completely of single bonds that remain hydrogen-saturated.
- Acyclic saturated hydrocarbons, often known as alkanes, have the general formula CnH2n+2The more general formula is CnH2n+2(1-r) , where r is the number of rings.
- There are no double or triple bonds between the carbon atoms in saturated hydrocarbons, as well as all carbon atoms form four unique covalent bonds.
- The name “saturated” refers to the structure’s saturation of hydrogen atoms, that makes them the simplest as well as least polar organic molecules.
- Saturated hydrocarbons can be found in either a linear or branched form, depending on the structure’s complexity.
- These hydrocarbons are found predominantly in petroleum products as well as other fossil fuels.
- Since saturated hydrocarbons burn with a blue, non-sooty flame, they are employed as fuel in cars as well as other engines.
- Saturated hydrocarbons exhibit the substitution process since they resist other reactions such as hydrogenation as well as oxidative addition.
- In comparison to unsaturated hydrocarbons, saturated hydrocarbons have a lower carbon concentration since the number of hydrogen atoms is relatively high.
- They’re also more stable as well as less reactive, as well as they can withstand nucleophile as well as electrophile attacks.
- Saturated hydrocarbons are divided into two groups: acyclic alkanes as well as cycloalkanes.
- Saturated hydrocarbons include methane, butane, propane, as well as cyclohexane, among others.
Definition of unsaturated hydrocarbons
Unsaturated hydrocarbons are hydrocarbons that have double or triple bonds between their carbon atoms.
- Alkenes, that have the general formula CnH2n , are unsaturated hydrocarbons with double bonds, whereas alkynes, that have the general formula CnH2n-2 , are unsaturated hydrocarbons with triple bonds.
- The word unsaturated refers to the fact that the molecules can be saturated by adding extra hydrogen atoms.
- Unsaturated hydrocarbons, like saturated hydrocarbons, can be found in straight-chain linear, branched, or aromatic forms.
- Since aromatic rings are created by the delocalization of double bonds between numerous carbon atoms, all aromatic hydrocarbons are unsaturated hydrocarbons.
- Unsaturated hydrocarbons, with the exception of aromatic compounds, are more reactive as well as undergo numerous addition reactions on multiple bonds.
- The degree of unsaturation, that is a measure of the amount of -bonds present in a molecule, can be used to express the unsaturation of hydrocarbons.
- Since there are fewer hydrogen atoms in unsaturated hydrocarbons, they have a higher carbon concentration than saturated hydrocarbons.
- Since unsaturated hydrocarbons have a lower hydrogen content, they produce a yellow flame, that reduces the flame’s moisture content.
- Since unsaturated hydrocarbons, like most hydrocarbons, are non-polar, their structures are held together by a weak van der Waal’s attraction.
- Unsaturated hydrocarbons have a distinct nomenclature than saturated hydrocarbons since the position of the double or triple bonds is indicated by adding numbers to the prefix.
- Ethane, acetylene, benzene, butadiene, as well as other unsaturated hydrocarbons are examples.
Key Differences between Saturated and Unsaturated Hydrocarbons
(Saturated vs Unsaturated Hydrocarbons)
[ninja_tables id=”5701″]
Saturated Hydrocarbons Examples
Methane
- Methane is the most basic aliphatic hydrocarbon, consisting of a single carbon atom bound to four hydrogen atoms in a single bond.
- It has the chemical formula CH4 as well as is a saturated hydrocarbon with all four covalent bonds attached to four separate hydrogen atoms.
- Methane is a simple hydrocarbon that burns with a pale blue flame that is mildly bright. It’s less combustible as well as more stable than other comparable chemicals.
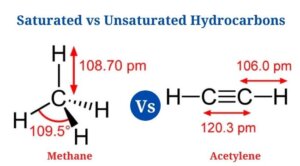
- In methane, all four carbon-hydrogen bonds have a bond length of 1.09 * 10 -10 as well as a bond angle of 109.5° . It has three sp3 hybridized orbitals as well as is a tetrahedral molecule.
- Methane is a valuable fossil fuel that is widely used as a biogas for cooking. It’s also a key member of the greenhouse gas family, as well as it’s one of the main contributors to global warming.
Unsaturated Hydrocarbons Examples
Acetylene
- Acetylene, often known as ethyne, is a two-carbon unsaturated hydrocarbon having the formula C2H2.
- It’s an alkyne with a triple carbon-to-carbon link as well as a single carbon-to-hydrogen covalent bond.
- Since the carbon-carbon triple bond places all four atoms in the same straight line with a bond angle of 180°, the molecule has a linear structure.
- One of the triple bonds is a -bond, while the other two are -bonds that are weaker.
- Oxyacetylene gas welding as well as cutting provide about 20% of the total acetylene found in the environment.
- Acetylene’s carbon-carbon triple bond is energy rich, allowing it to be employed as a good substrate for bacteria.
Saturated Hydrocarbons vs Unsaturated Hydrocarbons Citations
- Gautam AD, Pant M and Adhikari NR (2017). Comprehensive Chemistry Part 2. Heritage Publishers and Distributors. Kathmandu, Nepal.
- Methane” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Methane. Accessed 21 February, 2021.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6326, Acetylene. https://pubchem.ncbi.nlm.nih.gov/compound/Acetylene. Accessed Apr. 8, 2021.
- https://pediaa.com/difference-between-saturated-and-unsaturated-hydrocarbons/
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example