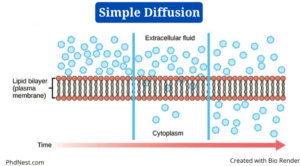
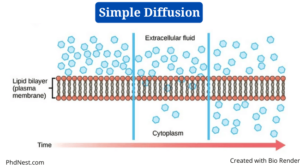
Simple Diffusion Definition
Simple diffusion is a sort of passive transport that happens when the electrochemical potentials of a solute on opposite sides of a permeable barrier differ.
- Diffusion refers to the “spreading out” of molecules from a higher concentration in other science areas such as chemistry, but in biology, the process involves a selectively permeable biological membrane.
- The movement of molecules happens along the concentration gradient in simple diffusion, as in all other passive transport modes, until the concentration of solute is uniform on both sides.
Principle of Simple Diffusion
How does simple diffusion work?
- Solute particles move around randomly Brownian motion in free diffusion via a membrane, just as they do in free solution.
- These different motions are essentially the outcome of the solute flow, which is a measured and repeatable quantity.
- Despite the fact that the path of a single solute particle cannot be anticipated, the aggregate of a large number of these trajectories can be.
- Teorell (1953) proposed the following formula to express the flux in free diffusion:
Flux = Mobility * Concentration * Driving Force
- The flux is defined as the number of moles of solute that pass a square centimetre of membrane in one second.
- The product of solute mobility, which evaluates the ease of transport, concentration, which measures the amount of material accessible to participate in the process, and driving force determines the flux.
- When the solute’s chemical potential in the two phases bounding the membrane is equal and its flux over the membrane is zero, the solute is in equilibrium.
- When the concentrations are different, however, a chemical gradient potential develops, which acts as a driving factor for the solute flux.
- The mobility of the solute, in turn, is determined by the membrane’s permeability for that particular solute, which must be determined experimentally in biological systems.
Diffusion of Electrolytes
- An extra force, in addition to the concentration gradient, drives the diffusion of charged species.
- When electrostatic potential gradients exist, charged solutes are subjected to electrical forces.
- As a result, rather than the chemical potential, the gradient of the electrochemical potential provides the driving factor for electrolyte movement.
- There are always at least two solute species in any electrolyte solution since it must contain at least one anion and one cation, resulting in multiple fluxes.
Factors Affecting Simple Diffusion
Since various parameters influence the rate of diffusion, these parameters/factors have an impact on the diffusion mechanism.
1 . Concentration gradient
- The diffusion of a nonelectrolyte is propelled by a concentration gradient across a biological membrane.
- As a result, the bigger the concentration differential across the membrane, the faster the diffusion rate.
- As the distribution of molecules across the membrane becomes more uniform, the rate of diffusion slows.
- Once equilibrium is attained across the membrane, the diffusion process comes to a halt.
2. Mass/Size of the solute molecules
- The size of the molecules has an impact on the rate of diffusion across a biological membrane.
- If the molecules are big, they will have a harder time moving over the membrane, slowing the rate of diffusion.
- As a result, the rate of diffusion for smaller molecules is faster than for bigger molecules.
3. Temperature
- The temperature of the system has an impact on the simple diffusion process.
- The energy of the molecules increases as the temperature rises.
- Molecules with more energy can pass the barrier faster, while particles with less energy take longer.
4. Solubility
- The solubility of molecules in a medium influences the rate of particle diffusion.
- Lipid-soluble molecules can move fast across a lipid layer, such as the plasma membrane.
- Similarly, depending on the structure of the biological membrane, polar and non-polar molecules flow at various rates.
5. Solvent density
- As the density of the solvent increases, the rate of diffusion slows.
- In a dense solvent, the solute will have a difficult time moving around.
- The mobility of solute in the cytoplasm of the cell is influenced by the density of the solvent.
- The flow of molecules and gases is slowed as the density of the cytoplasm increases, and the opposite is true for less dense cytoplasm.
6. Surface area and thickness of the biological membrane
- As the surface area of the membrane rises, the rate of diffusion increases.
- Because mobility is one of the components that causes flux, increasing the surface area increases the permeability or mobility of the molecules.
- Similarly, when the membrane thickness increases, the rate of diffusion decreases.
Simple Diffusion Examples
Oxygen and Carbon dioxide
- The flow of gases over a membrane in animals is a famous example of simple diffusion.
- To exchange oxygen and carbon dioxide dissolved in the blood, simple diffusion is used.
- The concentration gradient of these gases in the cells determines the direction of gas migration in the cells.
- The oxygen concentration in the alveoli is higher than in the blood vessels during inhaling. Also as a consequence, oxygen enters the bloodstream from the alveoli.
- Similarly, during exhalation, the concentration of carbon dioxide in the blood is higher than in the alveoli, causing carbon dioxide to flow towards the lungs.
- Gases are exchanged between blood and cells in a similar manner.
Movement of waste materials
- Simple diffusion is used to remove waste products in animals.
- The waste substance urea is discharged onto the blood by a simple diffusion process in the liver.
- In the kidneys, simple diffusion is employed to eliminate waste chemicals and toxins as well as absorb water. A separate active transport system exists in specific parts of the kidneys.
Nutrition in bacteria
- Bacteria and other prokaryotes lack a particular system for transporting nutrients, water, gases, and other solutes throughout their bodies.
- As a result, to move these molecules across the cytoplasm, they rely on simple diffusion.
- Furthermore, simple diffusion, which happens through the broad body surface, aids the excretion of waste products in bacteria.
Application of Simple diffusion
Simple diffusion is used in a variety of disciplines, including food, medicine, and the environment.
- The diffusion of gases and chemicals from tea leaves is important in the creation of a specific taste in beverages like tea and soda.
- In the action of medications in the body, the simple diffusion method is used. After ingesting a drug, the molecules are released into their various sites of action through a simple diffusion mechanism.
- Another example of a diffusion-related problem is air pollution. Air pollution is caused by the diffusion of numerous gases emitted by agricultural, industrial, and mechanical processes.
- Diffusion is also responsible for the production of alloys. When one metal is exposed to another for a long time, the atoms diffuse from one metal to the other, filling the gaps. As a result, a variety of alloys are formed.
Click Here for Complete Biology Notes
Simple Diffusion Citations
- https://socratic.org/questions/what-factors-affect-the-rate-of-diffusion-2
- https://sciencing.com/four-things-affect-rate-diffusion-8348637.html
- https://en.wikipedia.org/wiki/Passive_diffusion
- https://en.wikipedia.org/wiki/Electrochemical_potential
- https://answers.yahoo.com/question/index?qid=20151016202439AAVT1Q9
- https://www.sciencedirect.com/science/article/pii/B9780121191030500101
- https://www.merckmanuals.com/en-ca/home/lung-and-airway-disorders/biology-of-the-lungs-and-airways/exchanging-oxygen-and-carbon-dioxide
- https://www.getnitrogen.org/pdf/graham.pdf
- https://www.chegg.com/homework-help/questions-and-answers/facilitated-diffusion-type-passive-transport-allows-solutes-move-across-plasma-membrane-ce-q17996056
- https://s4.lite.msu.edu/res/msu/botonl/b_online/library/crofts/bioph354/lect9.html
- https://quizlet.com/83982729/chapter-16-chemistry-flash-cards/
- https://quizlet.com/6023729/ap-biology-chapter-07-membrane-structure-and-function-flash-cards/
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results